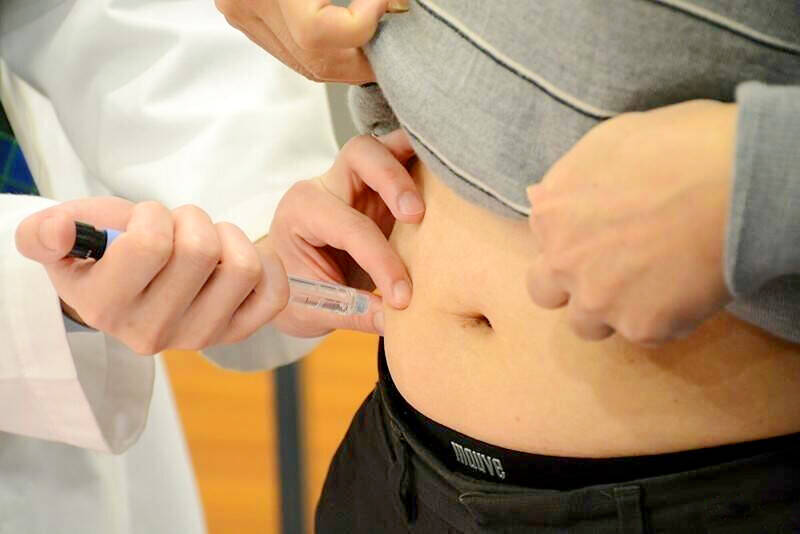
Taiwan FDA Warns: GLP-1 Weight Loss Drugs Like Ozempic Linked to 21 Adverse Reactions
In recent alerts on GLP-1 weight loss drugs side effects, Taiwan's Food and Drug Administration (FDA) emphasized that medications such as Ozempic are prescription drugs for weight management that must be prescribed by a doctor. These GLP-1 receptor agonists, originally developed for diabetes treatment, mimic the GLP-1 hormone to lower blood sugar levels and promote prolonged satiety. Over the past three years, the FDA has received 21 reports of suspected adverse reactions, highlighting the need for cautious, medically supervised use.
Understanding GLP-1 Receptor Agonists and Their Role in Weight Loss
GLP-1 receptor agonists, commonly referred to as "slimming injections (瘦瘦針)" in Taiwan, work by imitating the glucagon-like peptide-1 (GLP-1) hormone. This hormone, naturally produced in the gut, regulates blood sugar by stimulating insulin release, slowing gastric emptying, and signaling fullness to the brain. For weight loss, these effects reduce appetite and calorie intake, leading to significant reductions in body weight when used alongside diet and exercise.
Taiwan has approved only three GLP-1 medications for weight control: tirzepatide, semaglutide (found in Ozempic), and liraglutide. These are primarily for adults meeting specific criteria, such as a body mass index (BMI) above defined thresholds indicating obesity. In some cases, they may be prescribed to adolescents aged 12 and older following thorough medical assessment.
How GLP-1 Drugs Differ from Traditional Weight Loss Methods
Unlike stimulants or surgical options, GLP-1 agonists target metabolic pathways directly. Clinical trials have shown average weight loss of 15-20% of body weight over 68 weeks with semaglutide, far surpassing lifestyle interventions alone. However, their gastrointestinal mechanism—slowing digestion—also underlies many side effects, making patient selection and monitoring essential.
Reported Adverse Reactions in Taiwan: Key Facts from the FDA
Between January 1, 2023, and December 31 of the previous year, Taiwan's National Adverse Drug Reaction Reporting System logged 21 reports related to these GLP-1 drugs. Common symptoms included:
- Gastrointestinal disorders
- Hypoglycemia (low blood sugar)
- Nausea
- Vomiting
- Injection site discomfort
The FDA noted no abnormalities in overall drug safety or emergence of new risks based on these reports. Still, it issued a news release reminding the public to use these medications responsibly, evaluated and prescribed by a physician, and dispensed by a pharmacist.
"The 'slimming injections' are prescription drugs that must be evaluated and prescribed by a physician and dispensed by a pharmacist," the FDA stated. "People should not purchase such products online or through unverified channels, as counterfeit or substandard drugs, along with improper use, not only fail to help them lose weight but can also jeopardize their health."
Global Context: US Lawsuits and UK Guidance Updates
Experiences may vary, as seen abroad. In the US, thousands of users have reported serious side effects from GLP-1 weight loss drugs, leading to lawsuits. A USA Today story on January 28 detailed at least 4,400 patients in consolidated litigation against drugmakers since the first lawsuit in 2023, alleging inadequate warnings about severe side effects.
The UK's Medicines and Healthcare products Regulatory Agency updated its guidance on January 29, stating that "acute pancreatitis is a known, but infrequent side effect of taking GLP-1s," with "in some extremely rare cases the complications of acute pancreatitis can be particularly severe." These international developments prompted Taiwan's FDA to reinforce local vigilance.